MYODM-FSMP Pilot Interventional Clinical Study
The clinical study, which took place at Donostia University Hospital–Neuromuscular Unit, Department of Neurology, aimed to provide human data documenting the effects on HRQoL, fatigue, and hypersomnia of the food product formulated with caffeine and theobromine in the nutritional management of people living with DM1. It was conducted to support the health claims of the AUME product (food for special medical purposes) as part of a doctoral thesis that received the highest possible qualification from the examination board.
A total of 30 subjects participated in this study, aged between 28 and 72 years (mean age, 46±10), of both sexes (37% women, 63% men), all with a prior diagnosis of DM1, with an average CTG repeat count of 602±73 and mild to moderate proximal weakness (MIRS 3.0±1.1).
Although the investigational product was well tolerated by the vast majority of patients, 20% of participants withdrew from the study before the six-month period due to an external factor, the COVID-19 pandemic, which disrupted the normal progression of the pilot clinical trial. Even so, the study cohort was sufficient to extract results, particularly in men:
- 75% of the male patients who completed the active arm treatment reduced their daytime sleepiness, with an average improvement of 51%. This effect was statistically significant in men, whereas interestingly it was not observed in women, likely due to the group being too small for statistical purposes. Nevertheless, considering both genders, 63% of patients who completed the active arm treatment reduced their daytime sleepiness, with an average improvement of 33%.

Figure 3 graphically represents the comparisons of ESS (Epworth Sleepiness Scale) scores of the male patients in the active arm at the end of the study (6 months) compared to the baseline situation. Scores range from 0 to 24, where 0-6 is considered normal, 7-8 moderate sleepiness, and 9-24 abnormal/pathological sleepiness.
- Regarding motor function, men in the active arm experienced a statistically significant improvement in the 6MWT scale (six-minute walk test), with an average increase of 53 meters, equivalent to a 16% improvement from the initial visit.
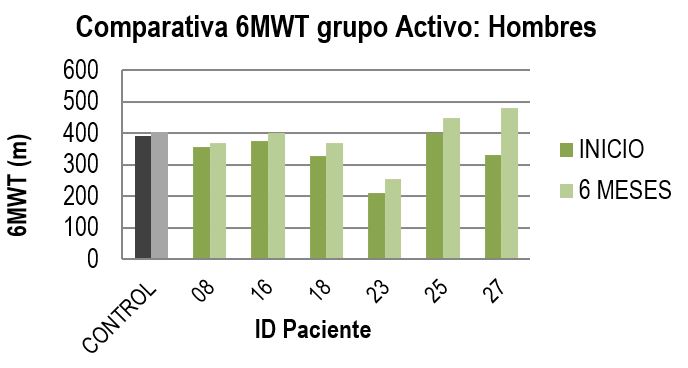
In the case of women, the results were not statistically significant. In the overall results for patients who completed the treatment considering both genders, a 12% improvement compared to the start of the study was observed.

